Nuclear energy produced by using a Nuclear Reactor is a power generation environment where a Control Nuclear Fission reaction is used to produce power.
Nuclear energy, a powerful and relatively low-carbon source of electricity, is produced through a complex process known as nuclear fission. This process involves splitting the nuclei of atoms, usually uranium-235 or plutonium-239, to release a tremendous amount of energy.
The energy generated is then converted into electricity through a series of well-orchestrated steps, including heat generation, steam production, and turbine rotation. In this post, we’ll take you through a detailed, step-by-step guide to understanding how nuclear energy is produced, from fuel preparation to the management of radioactive waste.
We built the first Nuclear Reactor in Chicago, USA in 1942.
Components of Nuclear Reactor
- The major parts of the Nuclear Reactor are:
- Fuel
- Moderator
- Control Rod
- Coolant
- Protection Wall
1. Fuel
Nuclear fuel refers to the material used in nuclear reactors to sustain a chain reaction. The most commonly used nuclear fuels are uranium and plutonium. These elements contain isotopes that can be split into smaller parts when struck by a neutron, releasing a tremendous amount of energy in the process.
It is a fissile material that is the fuel for Nuclear reactors. The most commonly used Nuclear Fuel is Uranium.
Example of Nuclear Fuel
- Uranium-235: The most prevalent nuclear fuel, uranium-235 is a naturally occurring isotope of uranium. It is favoured for its ability to sustain a nuclear chain reaction.
- Plutonium-239: Created from uranium-238 in a reactor, plutonium-239 is another isotope capable of sustaining fission. It is commonly used in mixed oxide (MOX) fuel, which combines plutonium and uranium.
- Thorium-232: An emerging nuclear fuel, thorium-232 can be converted into uranium-233 through a neutron absorption process. It offers a potentially safer and more abundant alternative to uranium and plutonium.

2. Moderator
A moderator in a nuclear power plant is a material used to slow down fast neutrons produced during nuclear fission. The slowing down of neutrons is crucial because slow (thermal) neutrons are more likely to cause further fission in fuel nuclei, such as uranium-235 or plutonium-239, sustaining the chain reaction needed for continuous energy production.
2.1 Function of Moderators
2.1.1 Slowing Down Neutrons
Neutrons released during fission are initially very fast and have high energy. Moderators reduce the speed and energy of these neutrons, making them more effective in sustaining the chain reaction.
2.1.2 Increasing Fission Probability
Slow (thermal) neutrons have a higher probability of being captured by the fuel nuclei, thereby increasing the likelihood of further fission events.
2.1.3 Increasing Fission Probability
Slow (thermal) neutrons have a higher probability of being captured by the fuel nuclei, thereby increasing the likelihood of further fission events.
2.1.4 Maintaining Reactor Stability
By ensuring a steady rate of fission, moderators help maintain the stability and control of the nuclear reactor, preventing runaway reactions or shutdowns.
2.2 Types of Moderators
2.2.1 Water
Light water (ordinary H2O) is the most common moderator used in nuclear reactors, especially in Pressurized Water Reactors (PWR) and Boiling Water Reactors (BWR). It serves the dual purpose of acting as a coolant and a moderator.
2.2.2 Heavy Water
Heavy water (D2O) contains deuterium, an isotope of hydrogen, and is used in reactors. Heavy water is an excellent moderator because it is less likely to absorb neutrons than light water.
2.2.3 Graphite
Graphite is a form of carbon used as a moderator in reactors like the RBMK (Reaktor Bolshoy Moshchnosti Kanalny) and some gas-cooled reactors. It effectively slows down neutrons without capturing them.
2.2.4 Beryllium
Beryllium is a lightweight metal that can also serve as a moderator. It is less common but used in some research reactors due to its low neutron absorption properties.
2.3 Importance of Moderators
2.3.1 Control of Fission Reaction
Moderators are essential for controlling the fission reaction rate, ensuring that it proceeds at a stable and manageable rate.
2.3.2 Efficiency and Safety
Proper moderation increases the efficiency of the nuclear reactor, leading to higher energy output and reduced fuel consumption. Additionally, effective moderation enhances the overall safety of the reactor by preventing uncontrolled reactions.
3.Control Rod
Control rods are used to control the number of neutrons to control the chain reaction. The most widely used control rods are Boron or Cadmium.
Control rods are long, slender tubes made of neutron-absorbing materials. They are inserted into the nuclear reactor core alongside fuel rods. Their primary function is to control the rate of the nuclear fission reaction by absorbing excess neutrons, which are essential to sustaining the chain reaction.
3.1 Function of Control Rods
3.1.1 Regulating the Reaction
By absorbing neutrons, control rods reduce the number of neutrons available to cause further fission. This allows operators to adjust the reactor’s power output as needed.
3.1.2 Shutting Down the Reactor
In the event of an emergency or planned shutdown, control rods are fully inserted into the reactor core to absorb a maximum number of neutrons, effectively halting the fission reaction.
3.1.3 Maintaining Stability
By adjusting the position of control rods, operators can maintain a stable and controlled fission reaction, preventing both runaway reactions and unplanned shutdowns.
3.2 Materials Used in Control Rods
Control rods are made from materials with a high capacity for absorbing neutrons. Common materials include:
- Boron: Often used in the form of boron carbide (B4C), boron is an effective neutron absorber.
- Cadmium: Cadmium rods are efficient at absorbing thermal neutrons and are commonly used in many reactor designs.
- Hafnium: Hafnium is another material known for its neutron absorption properties and is used in some reactors.
- Silver-Indium-Cadmium (Ag-In-Cd) Alloy: This alloy combines the properties of silver, indium, and cadmium to create highly effective control rods.
4. Coolant
A coolant is a substance used to remove heat from the reactor core and transfer it away to a secondary system where it can be used to generate electricity or be dissipated. Coolants ensure that the reactor operates within safe temperature limits, preventing damage to the reactor and maintaining the integrity of the fuel.
4.1 Function of Coolants
4.1.1 Heat Removal
The primary function of a coolant is to absorb the heat produced during nuclear fission and transport it away from the reactor core.
4.1.2 Temperature Regulation
Coolants help regulate the temperature within the reactor, ensuring it remains within safe operational limits.
4.1.3 Steam Production
In many reactors, the heat carried by the coolant is used to produce steam, which then drives turbines to generate electricity.
4.1.4 Safety
By preventing overheating, coolants play a vital role in maintaining the safety and stability of the reactor.
4.2 Types of Coolants
4.2.1 Water
4.2.1.1 Light Water
Ordinary water (H2O) is the most common coolant used in nuclear reactors, particularly in Pressurized Water Reactors (PWR) and Boiling Water Reactors (BWR). It acts as both a coolant and a moderator.
4.2.1.1 Heavy Water
Heavy water (D2O) contains deuterium, an isotope of hydrogen. It is used in reactors like the CANDU (Canada Deuterium Uranium) reactors. Heavy water is an excellent moderator and coolant due to its low neutron absorption properties.
4.2.2 Gas
4.2.2.1 Carbon Dioxide (CO2)
Used in some gas-cooled reactors, carbon dioxide is an effective coolant due to its ability to transfer heat efficiently.
4.2.2.2 Helium
An inert gas with excellent heat transfer properties, helium is used in High-Temperature Gas-Cooled Reactors (HTGR) and other advanced reactor designs.
4.2.3 Liquid Metals
4.2.3.1 Sodium
Liquid sodium is used in fast breeder reactors due to its high thermal conductivity and ability to operate at high temperatures without pressurization.
4.2.3.2 Lead and Lead-Bismuth Eutectic
These liquid metals are used in some advanced reactor designs for their excellent heat transfer capabilities and high boiling points.
4.2.4 Molten Salts
Molten salts, such as fluoride or chloride salts, are used in Molten Salt Reactors (MSR) due to their ability to operate at high temperatures and their good heat transfer properties.
4.2.5 Importance of Coolants
4.2.5.1 Safety
Coolants are critical for maintaining the safe operation of the reactor by preventing overheating and potential meltdowns.
4.2.5.2 Efficiency
Effective heat removal and transfer by coolants ensure that the reactor operates efficiently, maximizing energy production.
4.2.5.3 Versatility
Different types of coolants allow for various reactor designs, each with specific advantages and applications.
5. Protection Wall
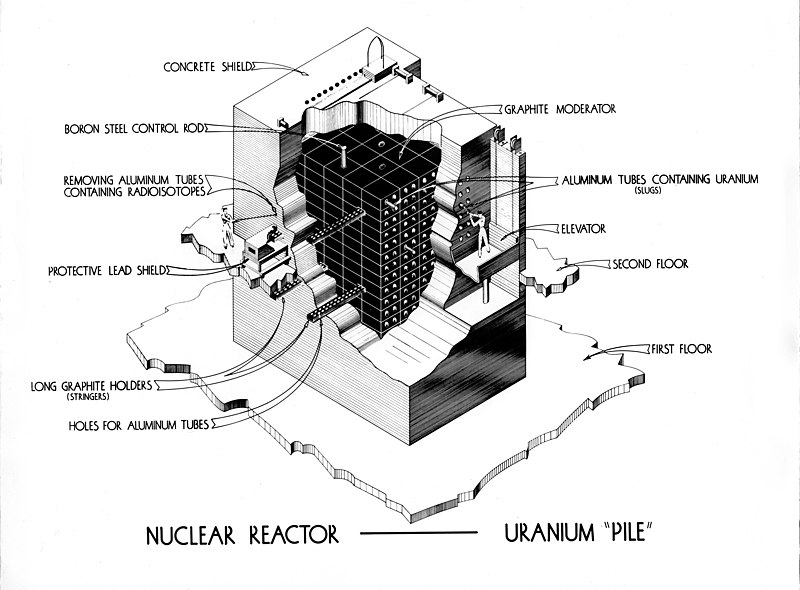
Protection walls, also known as containment structures or barriers, are critical safety features in nuclear power plants. These walls are designed to contain the release of radioactive materials and protect the environment and people from potential hazards. In this blog post, we will explore the function, types, and importance of protection walls in nuclear power plants.
5.1 Function of Protection Walls
5.1.1 Containment
Protection walls are designed to contain any radioactive materials that might be released during normal operation or in the event of an accident. This ensures that harmful radiation does not escape into the environment.
5.1.2 Shielding
These walls provide shielding against radiation, protecting plant workers and the surrounding community from exposure.
5.1.3 Structural Support
Protection walls also offer structural support to the reactor building, enhancing the overall integrity and stability of the facility.
5.2 Types of Protection Walls
5.2.1 Primary Containment
The innermost barrier surrounding the reactor core, designed to contain radioactive materials under all conditions. It includes components like the reactor pressure vessel and the biological shield.
5.2.2 Secondary Containment
An additional layer outside the primary containment, providing extra protection. It typically includes reinforced concrete walls and steel liners.
5.2.3 Tertiary Containment
In some designs, a third layer of containment may be present to provide further security and redundancy.
5.3 Importance of Protection Walls
5.3.1 Safety
The primary purpose of protection walls is to ensure the safety of the plant workers, the public, and the environment by preventing the release of radioactive materials.
5.3.2 Regulatory Compliance
Protection walls help nuclear power plants meet strict safety and regulatory standards set by national and international authorities.
5.3.3 Public Confidence
The presence of robust containment structures enhances public confidence in the safety of nuclear power plants.
Nuclear energy is commonly measured in Joules.
Uses of Nuclear Reactor
- Used for power generation
- Used to produce Radio Isotopes that used for various application such as Medicine, Industries, etc.
- Used for research in Nuclear Physics.
- Used to convert non-fissionable material into fissionable material.
Types of Nuclear Reactor
- Breeder reactor
- Fast Breeder Reactor
- Pressurized Water Reactor
- Pressurized Heavy Water Reactor
- Boiling Water Reactor
- Water-Cooled Reactor
- Gas-Cooled Reactor
- Fusion Reactor
- Thermal Reactor
Conclusion
Understanding the step-by-step process of how nuclear energy is produced provides insight into one of the most powerful and efficient means of generating electricity. From the initial fuel preparation and nuclear fission reaction to the final generation of electricity, each step is meticulously designed to harness the immense energy contained within atomic nuclei. The use of nuclear energy, despite its complexities, offers a sustainable and low-carbon alternative to traditional fossil fuels. As advancements continue in nuclear technology, ensuring safety and efficiency remains paramount, further solidifying its role in meeting the world’s growing energy demands. By appreciating the intricate details and safety measures involved, we gain a deeper respect for this remarkable source of power that lights up our homes and powers our industries.
